Photosynthesis is the process by which plants produce energy using sunlight. In artificial photosynthesis, man-made materials are used to mimic photosynthesis done by leaves.
A commonly used man-made material is titanium dioxide (TiO2, also called titania). The feature that makes TiO2 suitable for photosynthesis is its band gap. Titania has a band gap of 3.2 eV (electron volt). The separation energy of band gap corresponds to the ultraviolet energy of sunlight (wavelength of ~387 nm = 3.2 eV). Therefore, when titania is illuminated with sunlight, it absorbs untraviolet light in sunlight. As a result, electrons from its lower band (valence band) is promoted to the upper band (conduction band).
The cartoon below shows a typical photosynthesis reaction happening in titania photocatalyst.
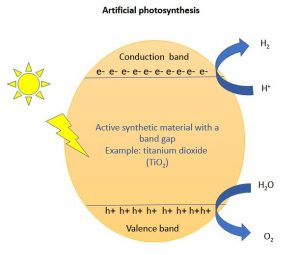
The free electrons thus accumulated in the conduction band has the potential to reduce H+ ions to H2 dihydrogen). Valence band holes (h+), in the meantime, oxidizes water molecules (H2O) to O2.
The H2 can then be used as a carbon-free fuel or as raw material in the production of many important chemicals.